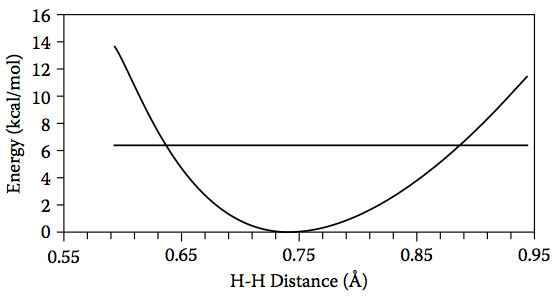
Figure 4.18. The relative energy of H2 as a function of H–H distance and the energy of the lowest vibrational level (horizontal line).
From Molecular Modeling Basics CRC Press, 2010
From Molecular Modeling Basics CRC Press, 2010
When discussing structure and other molecular properties it is important to remember that the molecule is in constant internal motion due to vibrational motion. The bond length and angles that one so carefully computes and reports to so many decimal places actually changes considerably with time.
Consider the H2 molecule. Using B3LYP/6-31G(d) and the harmonic oscillator approximation, the frequency for the H–H stretch vibration is 4450 cm–1, which corresponds to 6.4 kcal/mol of kinetic energy. If we compute the energy as a function of the H–H bond length (Figure 4.18), we see that this kinetic energy is enough to compress the H–H bond length to roughly 0.64 Å and increase it to 0.88 Å: the classical turning points.
The classical turning points can be estimated using the harmonic approximation of the potential energy surface (PES)
 where νi is the vibrational frequency in units of cm–1, with a corresponding normal coordinate li (see section 1.3 of the book), and n is the vibrational quantum number. Thus, νi = 4450 cm–1 corresponds to a turning point at li =±0.087 amu½ × Å which can be converted to Cartesian coordinates (and hence a bond length) using the normal mode component Lij
where νi is the vibrational frequency in units of cm–1, with a corresponding normal coordinate li (see section 1.3 of the book), and n is the vibrational quantum number. Thus, νi = 4450 cm–1 corresponds to a turning point at li =±0.087 amu½ × Å which can be converted to Cartesian coordinates (and hence a bond length) using the normal mode component Lij  In the case of H2, this gives classical turning points at 0.621 Å and 0.865 Å, which are in good agreement with the previous estimate from Figure 4.18. The screencast below shows ho MacMolPlt can be used to estimate the classical turning points.
In the case of H2, this gives classical turning points at 0.621 Å and 0.865 Å, which are in good agreement with the previous estimate from Figure 4.18. The screencast below shows ho MacMolPlt can be used to estimate the classical turning points.For lower frequencies the displacements at the classical turning points can be quite substantial, both because the PES is flatter and because higher vibrational levels are populated. For example, in the case of ethane the lowest frequency is 310 cm–1 [at the B3LYP/6-31G(d) level of theory] and corresponds mainly to rotation about the CC bond (Figure 4.20a). For the ground vibrational level the classical turning points occur at li =±0.330 amu½ × Å , which corresponds to a dihedral angle change of ±14° (Figure 4.20b, see screencast below). At room T 4% of the ethane molecules have three quanta of energy in this mode (n = 2), corresponding to a kinetic energy of 2.2 kcal/mol, enough to change the dihedral angle by ±31°.
Figure 4.20. (a) Normal mode associated with the lowest frequency computed for ethane using B3LYP/6-31G(d). (b) Structure obtained by displacing along the mode by 0.330.
Click on the picture for an interactive version.
Click here for a pop-up window
From Molecular Modeling Basics CRC Press, 2010
Click on the picture for an interactive version.
Click here for a pop-up window
From Molecular Modeling Basics CRC Press, 2010
Some of the lowest frequencies you’ll observe are for intermolecular interactions such as the water dimer hydrogen bond (Figure 4.22). Curious how much the geometry is displaced? Why not try it yourself? (See this post to get started with GAMESS).
Figure 4.22. (a) The PM3 normal mode corresponding to (a) torsional motion about the hydrogen bond and (b) hydrogen bond stretch in the water dimer. The corresponding frequencies are 128 and 415 cm–1.
Click on the picture for an interactive version.
Click here for a pop-up window
From Molecular Modeling Basics CRC Press, 2010
Click on the picture for an interactive version.
Click here for a pop-up window
From Molecular Modeling Basics CRC Press, 2010
Using MacMolPlt to compute the displaced geometry
As you can see from the second equation, the normal coordinate is basically a scale factor. Thus, MacMolPlt can be used to compute a displaced geometry by converting li to a percent and changing the units to amu½ × Bohr by dividing by 0.52918 Å/Bohr. Thus, li =±0.330 becomes 62.0% (note that there is a mistake in the book: I forgot about the unit conversion in Figure 5.27). Unfortunately, MacMolPlt does not include the m-½ so this is only an estimate.






2 comments:
I was reading your entry here and the book you wrote, and I was just trying to figure out where the factor of 1331 comes from. Could you please explain?
It's right there in Eq 4.10 of the book, and 1331 is explained in the sentence immediately after the Eq. So I am not sure what part you don't understand. Can you give me more details?
Post a Comment