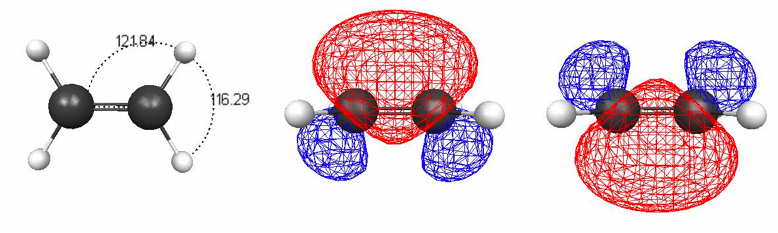
Figure 4.12. (a) B3LYP/6-31G(d) optimized geometry of ethene; (b) and (c) 0.045 au isosurfaces of the two localized banana MOs corresponding to the double bond.
Click on the picture for an interactive version
From Molecular Modeling Basics CRC Press, 2010
VSEPR theory can be used to explain structures of molecules with double and triple bonds, though this is rarely done in textbooks. Figure 4.12 shows some localized MOs for ethene, where the double bond is shown to consist of two curved MOs (sometimes called banana bonds), rather than the usual sigma and pi MOs. The banana LMOs are less spread out than single-bond LMOs, leading to less repulsion and an H–C–C angle larger than 109.5°, namely 116.3° [at the B3LYP/6-31G(d) level of theory].
Click on the picture for an interactive version
From Molecular Modeling Basics CRC Press, 2010
VSEPR theory can be used to explain structures of molecules with double and triple bonds, though this is rarely done in textbooks. Figure 4.12 shows some localized MOs for ethene, where the double bond is shown to consist of two curved MOs (sometimes called banana bonds), rather than the usual sigma and pi MOs. The banana LMOs are less spread out than single-bond LMOs, leading to less repulsion and an H–C–C angle larger than 109.5°, namely 116.3° [at the B3LYP/6-31G(d) level of theory].
Figure 4.13. 0.045 au isosurfaces of a localized pi MO [(a) top view and (b) side view] and two localized sigma bond MOs in benzene. The level of theory is B3LYP/6-31G(d).
Click on the picture for an interactive version
From Molecular Modeling Basics CRC Press, 2010
In the case of benzene the LMOs actually look like sigma and pi orbitals (Figure 4.13). Figure 4.13a and b are two views of the pi-bond LMO primarily between C4 and C5. Notice, however, that there is significant delocalization onto C3 and C6. There are identical pi-bond LMOs between C3 and C2 as well as C1 and C6, and the net result is an identical C–C bond length of 1.397 Å, roughly halfway between the CC bond length in ethane (1.531 Å) and ethene (1.331 Å).
I have discussed how to make these plots in a previous post.
From Molecular Modeling Basics CRC Press, 2010
In the case of benzene the LMOs actually look like sigma and pi orbitals (Figure 4.13). Figure 4.13a and b are two views of the pi-bond LMO primarily between C4 and C5. Notice, however, that there is significant delocalization onto C3 and C6. There are identical pi-bond LMOs between C3 and C2 as well as C1 and C6, and the net result is an identical C–C bond length of 1.397 Å, roughly halfway between the CC bond length in ethane (1.531 Å) and ethene (1.331 Å).
I have discussed how to make these plots in a previous post.





3 comments:
Hi! I'm a graduate student in Argentina, and I'm doing some quantum molecular dynamics simulations using GAMESS US (keyword runtyp=md + $MD group).
Do you know of any program or hack with which I could visualize the trajectory and/or (and at this point this would seem to be utopic) some actual data processing?
I tried macmolplt, but it turned out to be not very useful.
So far I managed to do some post processing writing perl scrips (for calculating dihedral angles from the cartesian coordinates of each frame, for example), but I would be very happy to know if there's any higher level tool I could use.
Thank you very much in advance!
Excellent blog btw! Keep it up!
Greetings
Diego J. Alonso de Armiño
Hi Diego,
This is not something I have direct experience with, but it is my impression that the VMD program is a popular trajectory analysis tool.
I am not sure if VMD can read in a GAMESS trajectory file, but if you know perl then it shouldn't be too hard to reformat it (or look intro whether Open Babel can handle this).
Hope this helps, and glad to hear you like the blog.
Jan,
I actually tried to use VMD, unfortunately it doesn't support GAMESS trajectory files nor output files from a MD simulation. Extended support for GAMESS is aparently planned for future versions of VMD, though.
I think I'll do what you suggest and try to make a conversion from the GAMESS format to some other which is supported by VMD.
Thank you very much for your help, and keep up the excellent work!
Diego
Post a Comment